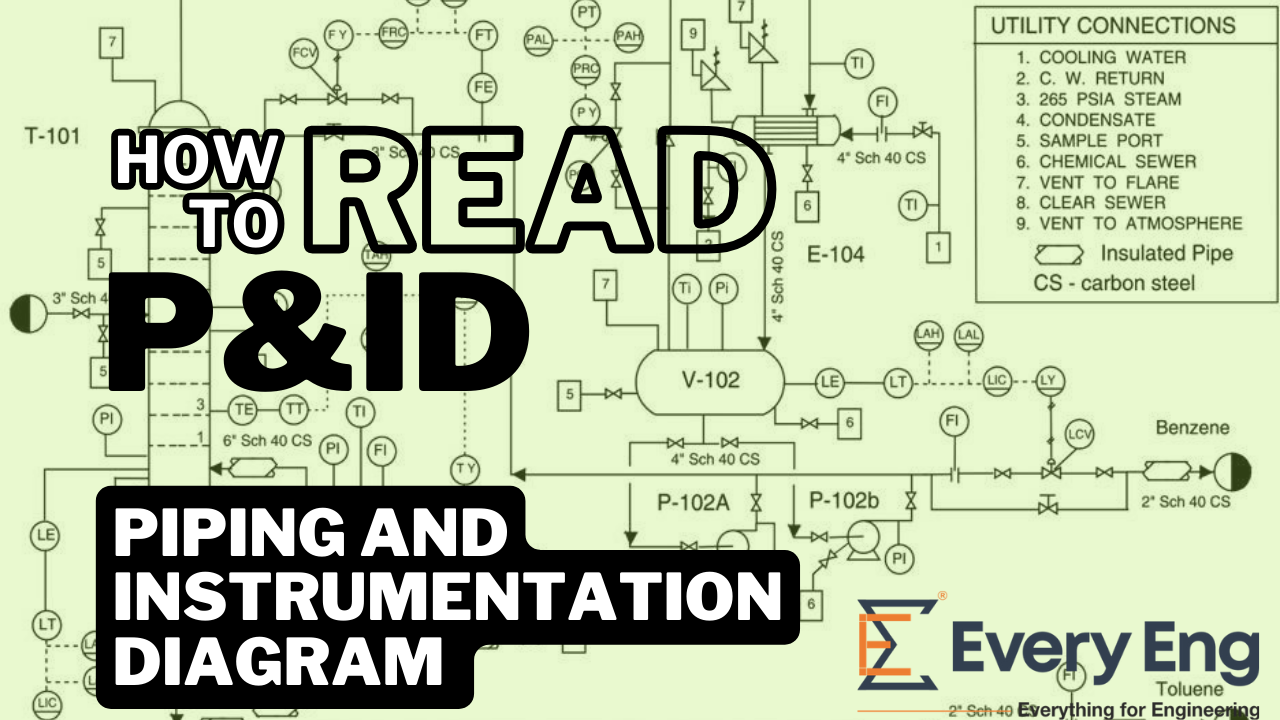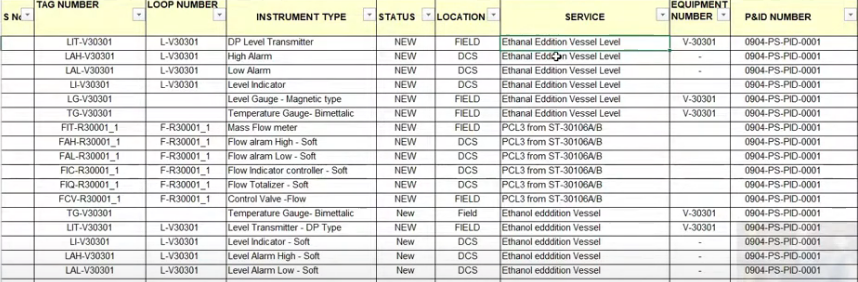
Physical Metallurgy - Learn about Iron (Fe) – Iron Carbide (Fe3C) Phase Diagrams
$ 20
Why enroll
Course details
Course suitable for
Key topics covered
Training details
This is a live course that has a scheduled start date.
Live session
Our Alumni Work At
Why people choose EveryEng
Industry-aligned courses, expert training, hands-on learning, recognized certifications, and job opportunities—all in a flexible and supportive environment.
- Industry Veteran
- Trainer Review

Petrofac
I was skeptical at first, but EveryEng's training programs really delivered. I gained the skills and confidence to take on challenging projects and advance my career. Highly recommended!

ITER
The user experience on EveryEng is fantastic! The platform is simple, efficient, and provides excellent educational content. It helps developing new skills and gain confidence in engineering career.

Subsea7
I was searching for a reliable platform to expand my engineering knowledge, and EveryEng exceeded my expectations. The content and courses are well-structured, informative, and taught by experienced professionals. A great platform for Engineers!

Technip
The content quality on EveryEng is outstanding! Every lesson is well-organized, and the instructors explain everything so clearly. This platform can help every engineer grow professionally in ways I never imagined.

SLB
I never imagined an online learning platform could be this effective! EveryEng’s courses are top-notch, the mentors are industry experts, and the skills my team gained have made a real difference in the performance!"
Questions and Answers
No questions yet - Be the first one to ask!