Unit Operations - Basic to Advance
FREE
21 already enrolled!
Beginner course for learners
Foundational Learning
Access to Study Materials
Self-Paced Learning

Unit Operations - Basic to Advance
Trainers feedback
5
(1 reviews)
Course type
Watch to learn anytime
Course duration
240 Min
Course start date & time
Access anytime
Language
English
This course format through pre-recorded video. You can buy and watch it to learn at any time.
Opportunities that awaits you!

Earn a course completion certificate
Add this credential to your LinkedIn profile, resume, or CV. Share it on social media and in your performance review
Course content
The course is readily available, allowing learners to start and complete it at their own pace.
Unit Operations - Basic to Advance
2 Lectures
240 min
Unit Operations-1
Preview
144 min
Unit Operations-2
96 min
Course details
To equip participants with a thorough understanding of unit operations, covering fundamental concepts to advanced techniques, enabling them to design, analyze, and optimize various industrial processes.
Dive into the core principles of unit operations, explore key processes such as distillation, filtration, heat exchange, and fluid flow, and learn advanced methods for process optimization and troubleshooting through practical examples and case studies.
Course suitable for
Oil & Gas Pharmaceutical & Healthcare Energy & Utilities Chemical & Process Onshore Pipeline Petroleum
Key topics covered
Introduction to Unit Operations
Definition and significance in industrial processes
Overview of various unit operations
Fluid Mechanics
Fluid properties and behavior
Flow in pipes and channels
Pumps, compressors, and fluid movers
Heat Transfer
Conduction, convection, and radiation
Heat exchangers: types, design, and operation
Evaporation and condensation
Mass Transfer
Principles of diffusion and mass transfer
Distillation: methods and equipment
Absorption and stripping
Extraction: liquid-liquid and solid-liquid
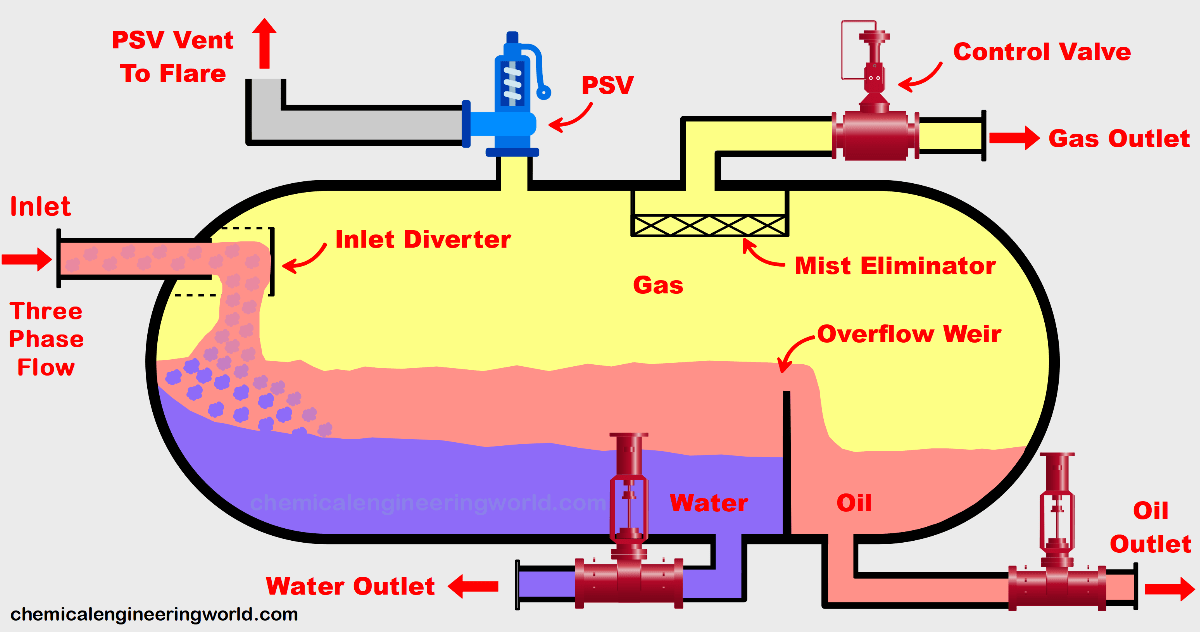
Mechanical Separations
Filtration: theory and equipment
Centrifugation
Sieving and screening
Chemical Reactors
Types of reactors: batch, continuous, and semi-batch
Reactor design and operation
Reaction kinetics and reactor sizing
Mixing and Agitation
Types of mixers and agitators
Mixing principles and scale-up
Applications in various industries
Crystallization and Solid-Liquid Separation
Principles of crystallization
Crystallizer design and operation
Solid-liquid separation techniques
Drying
Fundamentals of drying processes
Types of dryers and their applications
Design and optimization of drying systems
Membrane Processes
Membrane separation principles
Types of membranes and modules
Applications in industry
Advanced Topics in Unit Operations
Process intensification
Novel separation techniques
Environmental and energy considerations
Process Simulation and Modeling
Introduction to simulation software (e.g., Aspen Plus, COMSOL)
Building and analyzing models of unit operations
Practical examples and case studies
Optimization and Troubleshooting
Techniques for process optimization
Common operational issues and solutions
Case studies of real-world troubleshooting
Why people choose EveryEng
Industry-aligned courses, expert training, hands-on learning, recognized certifications, and job opportunities—all in a flexible and supportive environment.
- Industry Veteran
- Trainer Review

Process Engineering World
Questions and Answers
A: The rate of diffusion in gases is influenced by several factors including the concentration gradient, temperature, pressure, and the properties of the gases involved. According to Fick's law, the diffusion flux is proportional to the concentration gradient. Higher temperatures increase molecular motion, thus enhancing diffusion rates. Pressure can affect the gas density, which also impacts diffusion. Additionally, the molecular size and interactions between gas molecules play a significant role. For quantitative analysis, the Chapman-Enskog equation is used to estimate diffusion coefficients. For detailed information, refer to https://www.sciencedirect.com/topics/engineering/mass-transfer.
A: A distillation column separates components based on differences in their volatilities or boiling points. The mixture is heated to vaporize the more volatile component, which rises through the column. As the vapor ascends, it contacts descending liquid, facilitating mass and heat exchange. Components with lower boiling points concentrate in the vapor phase and are collected as the distillate, while those with higher boiling points condense and are removed as bottoms. The column efficiency depends on factors like the number of theoretical stages, reflux ratio, and tray or packing design. An excellent reference to understand distillation is Warren L. McCabe's 'Unit Operations of Chemical Engineering.' More info available at https://en.wikipedia.org/wiki/Distillation.
A: Heat transfer by conduction occurs through direct molecular collisions and energy transfer within a material without any movement of the material itself, such as heat flowing through a metal rod. Convection, on the other hand, involves heat transfer through the bulk motion of fluids (liquids or gases), where the fluid carries thermal energy as it moves. Convection can be natural (caused by buoyancy effects due to density differences) or forced (induced by external means like a pump or fan). Understanding these mechanisms helps in designing heat exchangers and thermal systems. More on this topic can be found at https://www.engineeringtoolbox.com/conduction-convection-radiation-d_430.html.
A: Unit Operations refer to the fundamental physical steps in any chemical engineering process, such as filtration, distillation, drying, evaporation, and crystallization. They are important because any complex chemical process can be broken down into these basic steps, allowing engineers to design, analyze, and optimize industrial systems efficiently. Understanding unit operations is crucial to developing scalable and efficient chemical processes. For more details, you can refer to Perry's Chemical Engineers' Handbook or visit https://en.wikipedia.org/wiki/Unit_operation.
A: Centrifugation is a separation technique that uses centrifugal force to accelerate the sedimentation of particles within a suspension. By spinning a sample at high speeds, denser particles are forced outward to the bottom of the centrifuge tube, separating from the liquid phase. This method is widely applied in chemical engineering to separate solids from liquids, clarify liquids, and in biochemical applications for separating cellular components. The separation efficiency depends on rotor speed, time, and particle size. For further reading, see https://www.britannica.com/science/centrifugation.
A: Absorption is a mass transfer process where a substance (the absorbate) penetrates into the bulk of another phase (the absorbent), like gas dissolving into a liquid. Adsorption involves the accumulation of molecules only on the surface of a solid or liquid, without entering the bulk phase, such as activated carbon capturing impurities on its surface. Both processes are used for separation and purification, but their mechanisms differ. Absorption involves solubility and diffusivity, while adsorption depends on surface area and chemical affinity. For more insights, see https://www.britannica.com/science/adsorption.
A: The flow of incompressible fluids in pipes is governed primarily by the Navier-Stokes equations, which describe momentum conservation, and the continuity equation for mass conservation. For practical engineering, these reduce to Bernoulli's equation for inviscid flow and the Darcy-Weisbach equation to calculate head loss due to friction. Engineers use these equations to design pipe networks, select pump sizes, and predict pressure drops. Moody charts are also commonly used to determine friction factors. For an in-depth explanation, check https://www.sciencedirect.com/topics/engineering/pipe-flow and https://www.engineeringtoolbox.com/pipe-pressure-loss-d_253.html.
A: Drying is the unit operation of removing moisture from solids by evaporation. It typically occurs in three stages: the initial warming period, the constant rate period, and the falling rate period. In the constant rate period, moisture evaporates from the surface at a constant rate while in the falling rate period, moisture removal becomes diffusion-limited as water evaporates from within the solid. Drying is critical in industries like pharmaceuticals, food, and chemicals to increase product shelf-life or prepare materials for further processing. Detailed physical models and drying kinetics can be found in 'Unit Operations in Food Processing' by K. S. Subrahmanyam. More info at https://www.ahrebs.com/blog/drying.
A: Pressure drop in packed columns refers to the loss of pressure as fluids flow through the packing material inside the column. It is caused by friction between the fluid and packing surfaces and the resistance to flow through the void spaces. Pressure drop is significant as it affects the hydraulic performance, energy consumption (pumping costs), and operational stability of processes like absorption and distillation. Excessive pressure drop can cause flooding or channeling in the column. Engineers use correlations such as the Ergun equation to estimate pressure drop in packed beds. A detailed explanation is available at https://www.cheresources.com/content/articles/pressuredroppackedbed.
More from Same Author
- Technical Courses
- Articles
5
5607
1
E-Learning
Unlimited access
Beginner
E-Learning
Unlimited access
Pre-recorded videos
5
1342
2
E-Learning
Unlimited access
Beginner
E-Learning
Unlimited access
Pre-recorded videos
5
1824
21
E-Learning
Unlimited access
Beginner
E-Learning
Unlimited access
Pre-recorded videos
Earning and Growth option in same Industry Domain
- Pre-recorded
- Online live session
- Offline
- Articles
4
4874
47
E-Learning
Unlimited access
Beginner
E-Learning
Unlimited access
Pre-recorded videos
2930
22
E-Learning
Unlimited access
Advanced
E-Learning
Unlimited access
Pre-recorded videos
2438
4
E-Learning
Unlimited access
Beginner
E-Learning
Unlimited access
Pre-recorded videos
More Training & Development option to expand your reach
- Technical courses
- Soft-skills courses
- Seminars
- Articles & Blogs
1711
6
Online
Live courses
December 31
160 Hrs
Advanced
Online
Live courses
Interacting with trainer
689
Online
Live courses
February 21
30 Hrs
Advanced
Online
Live courses
Interacting with trainer
4
256
1
Online
Live courses
February 28
2 Hrs
Beginner
Online
Live courses
Interacting with trainer