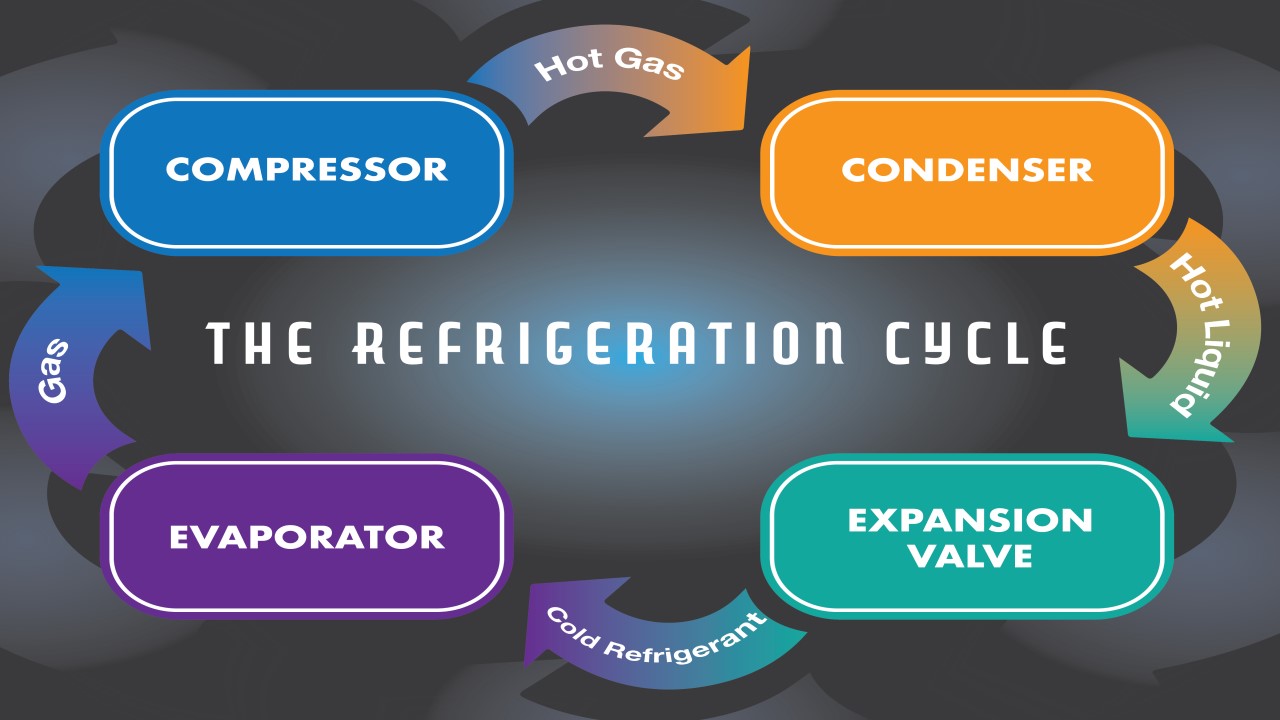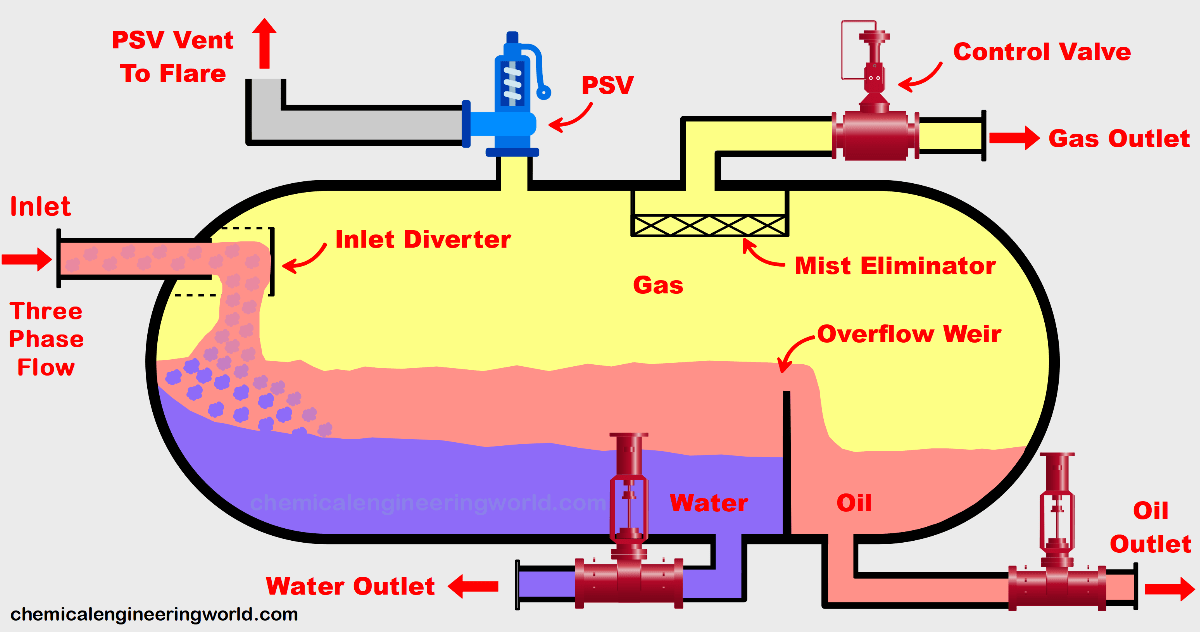
Physical Metallurgy - Understand Crystal Structures
$ 20
Opportunities that awaits you!

Earn a course completion certificate
Add this credential to your LinkedIn profile, resume, or CV. Share it on social media and in your performance review
Course details
Course suitable for
Key topics covered
Training details
This is a live course that has a scheduled start date.
Live session
Why people choose EveryEng
Industry-aligned courses, expert training, hands-on learning, recognized certifications, and job opportunities—all in a flexible and supportive environment.
- Industry Veteran
- Trainer Review
❮
❯